URGENT MEDICAL DEVICE CORRECTION
Labeling Correction for all TRUE METRIX® brand of Blood Glucose Monitoring Systems

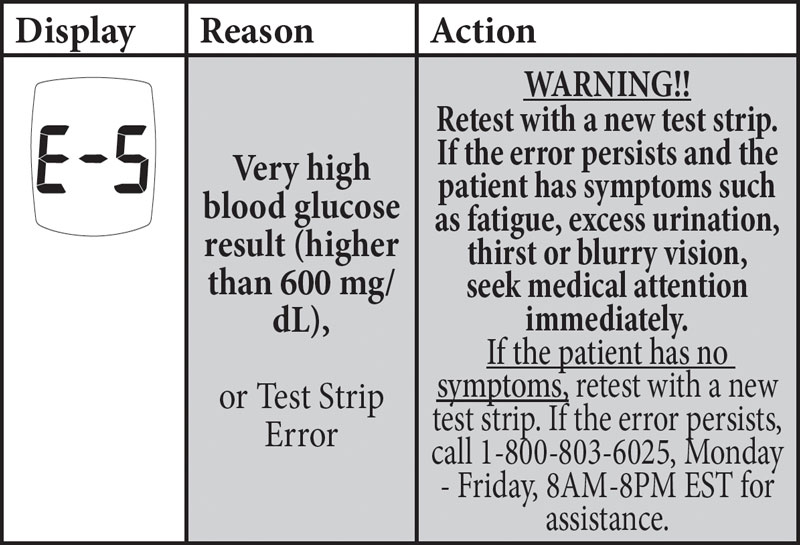
The correction involves the E-5 Error Code in the “Messages” section of the Owner’s Booklets/System Instructions for Use. The system displays an E-5 error code for a very high blood glucose event (> 600 mg/dL) or when there is a test strip error. As currently written, the instructions could potentially lead to a delay in treatment if the user does not seek medical attention immediately when they receive an E-5 error code and are experiencing symptoms of high glucose. A delay in treatment may result in serious adverse health consequences or death, especially for users with very high blood glucose levels.
Trividia is updating the E-5 Error Code actions to emphasize that users must seek medical attention immediately if they receive an E-5 error code and are experiencing symptoms of high glucose. Trividia Health will notify users of additional mitigation strategies as needed.
UPDATED E-5 INSTRUCTIONS for TRUE METRIX, TRUE METRIX AIR, and TRUE METRIX GO:

UPDATED E-5 INSTRUCTIONS for TRUE METRIX PRO:
You may continue to use the TRUE METRIX® Products. Products are not to be returned or replaced.
If you have any questions, please call Trividia Health Customer Care Department toll-free at 1-888-835-2723 Monday-Friday 8AM-8PM EST (excluding holidays)
This labeling correction affects all Products sold in the United States, United Kingdom, Mexico, Australia, and the Caribbean, including co-branded products sold under the following store or distribution partner names:
| Co-Branded Product Names |
|---|
| Care One (Ahold) |
| CenterWell (Humana) |
| CVS |
| Discount Drug Mart |
| Foster & Thrive/Sunmark/Healthmart (McKesson) |
| Good Neighbor Pharmacy (Cencora) |
| HEB |
| Henry Schein |
| HyVee |
| Kroger |
| Leader (Cardinal Health) |
| McKesson (Med Surg) |
| Meijer |
| ProCure (WynnMed) |
| Publix |
| Relion (Walmart) |
| Rite Aid |
| Signature Care (Albertsons) |
| Top Care (TopCo) |
| Walgreens |
| Farmacias Benavides (in Mexico) |
WHAT YOU SHOULD DO:
Trividia Health has notified the U.S. Food and Drug Administration (FDA) of this action.
If you would like to view additional information, please click here for the:
PRESS RELEASE
CUSTOMER COMMUNICATION
LIST OF IMPACTED PRODUCTS
Click here to view updated TRUE METRIX, TRUE METRIX AIR and TRUE METRIX GO Owner’s Booklets
Click here to view updated TRUE METRIX PRO Owner’s Booklet
Patient safety is our top priority, and we apologize for any inconvenience this correction may cause you.